Focus on the results of Pep2Dia® preclinical and clinical studies

Measuring, analysing and optimising the effectiveness of a bioactive before it is put on the market requires running preclinical and clinical studies.
It was after three years of research and testing carried out in collaboration with several laboratories that the Pep2Dia®bioactive was created, an innovative ingredient in the regulation of blood sugar.
Clinical and preclinical studies in collaboration with various partners
All of the steps leading to the development and testing of bioactive Pep2Dia® are the result of close collaboration between several partners: La Rochelle University, the LIENSs laboratory and the Ingredia company.
CNRS’s LIENSs laboratory (Littoral ENvironnement et Sociétés) is an interdisciplinary research unit working with La Rochelle University on the challenges of sustainable development in connection with the coast. It integrates the skills of many disciplines ranging from environmental sciences to humanities, but also includes chemistry and biotechnology.
« Proven effectiveness in three clinical studies »
Ingredia is a subsidiary of the « Prospérité Fermière » dairy cooperative, based in Pas-de-Calais. It specialises in the processing of milk and, in particular, the cracking of milk.
Thanks to its expertise and its partnership with research laboratories, Ingredia produces dairy ingredients and bioactives to promote better human nutrition.
Preclinical and clinical studies to demonstrate the safety and efficacy of Pep2Dia®
The development of a bioactive ingredient before it is marketed goes through several phases of testing, the role of which is to validate its efficacy and the absence of side effects.
Preclinical studies of Pep2Dia®
To understand and validate the mechanism of action of Pep2Dia®, a preclinical study was carried out.
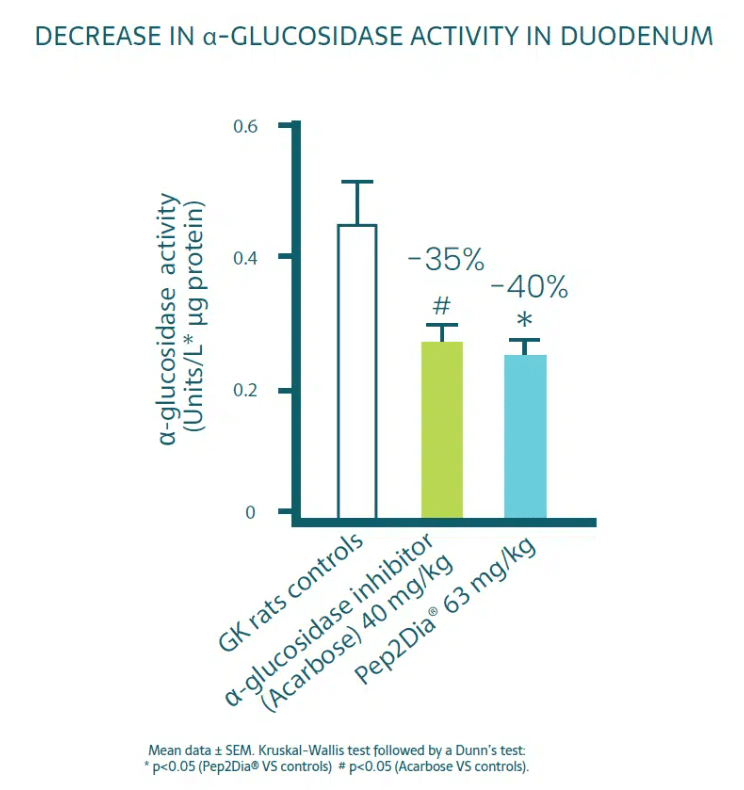
Pep2Dia® inhibits the action of a-glucosidase, an enzyme which converts poly and disaccharides into glucose.
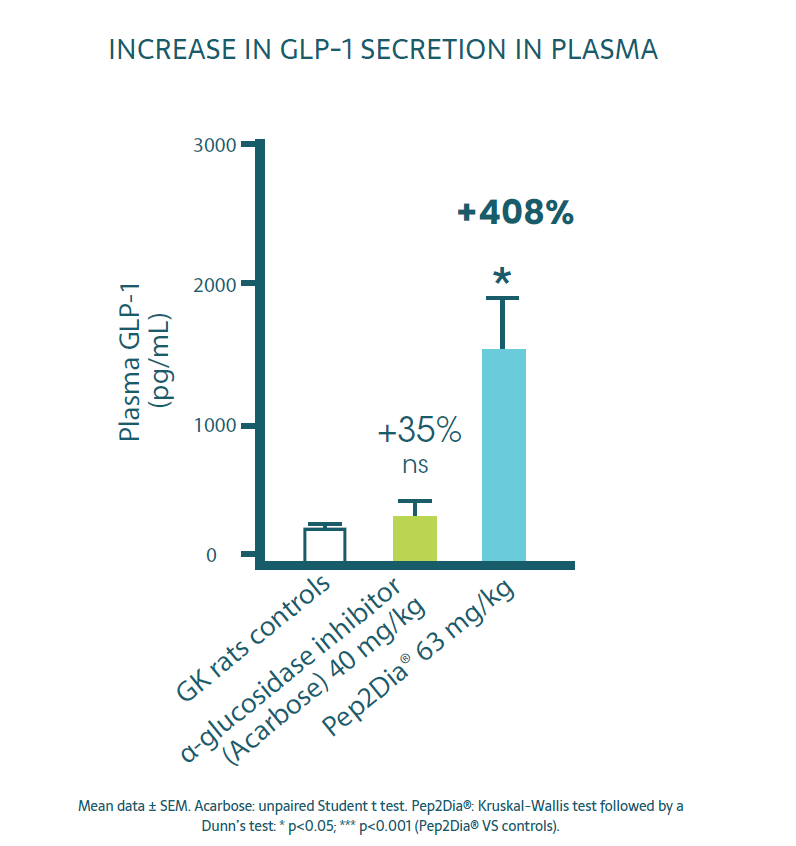
Pep2Dia® increases the secretion of GLP-1, a hormone involved in the protection of pancreatic function via :
- Insulin secretion and synthesis
- Proliferation of beta cells
- The feeling of satiety
- Delay in gastric emptying
Following preclinical studies, clinical studies were conducted in 2018 to validate efficacy in humans.
Clinical studies of Pep2Dia®
Then, Ingredia researchers have verified the efficacy of the bioactive ingredient and identified the effective dose in humans.
To achieve this, the clinical study was carried out in two phases with people whose blood sugar levels allowed them to be classified as ‘prediabetic’.
Male and female prediabetic volunteers were recruited according to precise criteria:
- an age between 30 and 70; the average age in this study was 62 ;
- a fasting blood glucose level of between 1 and 1.25 g/l and/or a glycated haemoglobin with an HbA1c level of between 5.7 and 6.4% (fasting blood glucose is an instantaneous measure of blood glucose status, whereas the HbA1c blood test assesses blood glucose balance over a period of around two to three months);
- a BMI (body mass index) of between 19 and 35 kg/m2 ;
The first clinical study, carried out in 2018, was conducted in 2 phases on 21 volunteers.
The first phase was a randomized double-blind crossover study (random selection of the product tested) with acute supplementation (the patient and the researcher or doctor were unaware of the results of the random selection).
As part of the trial, the product was administered to volunteers 15 minutes before a high-sugar breakfast.
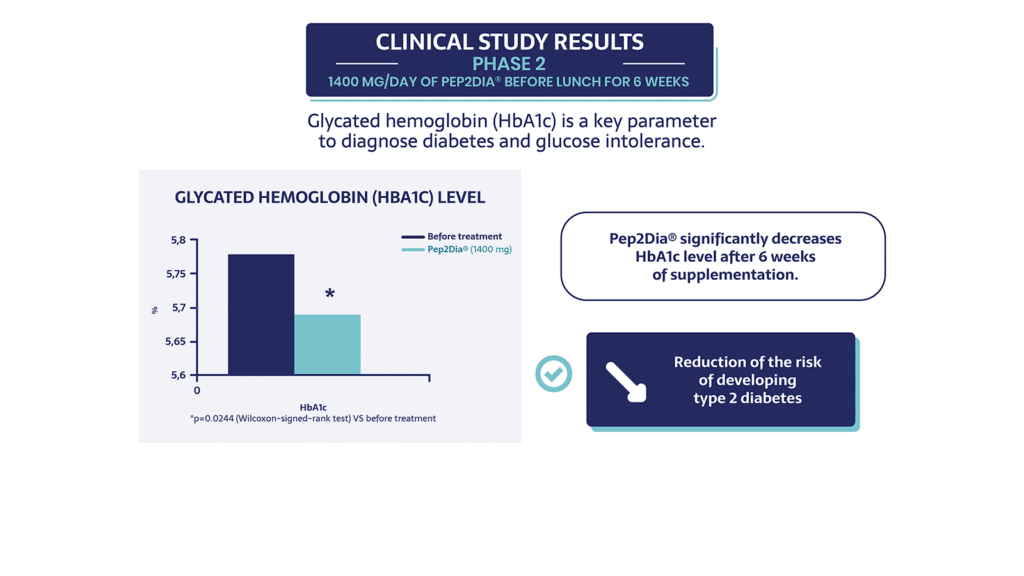
In the second stage of the clinical trial, a 1400 mg supplement of Pep2Dia® was administered for 6 weeks, before the main meal of the day.
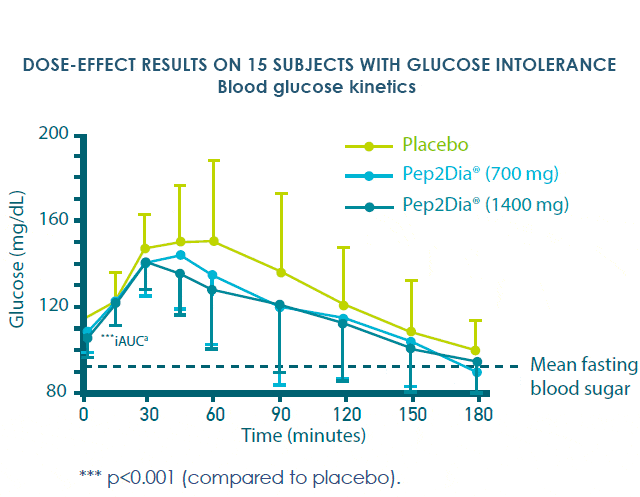
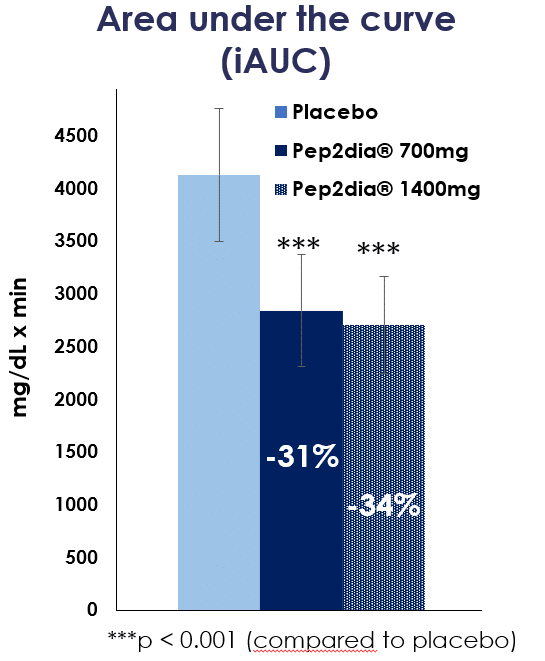
In 2020, another clinical study based on acute supplementation was carried out at doses of 700 and 1400 mg, using the same recruitment protocol but involving 36 volunteers.
The results of preclinical and clinical studies of Pep2Dia®
The 2018 study demonstrated a significant reduction in postprandial glycaemia, i.e. a reduction in blood glucose levels after meals, without affecting the increase in insulin secretion.
Pep2Dia® reduces blood sugar levels after a sweet meal by 21% in people with pre-diabetes.
Pep2Dia® also acts by lowering peak blood sugar levels after a meal rich in sugar (-7% on peak blood sugar levels compared with placebo). What’s more, Pep2Dia® does not increase insulin levels, which is important for people with prediabetes who tend to over-secrete insulin.
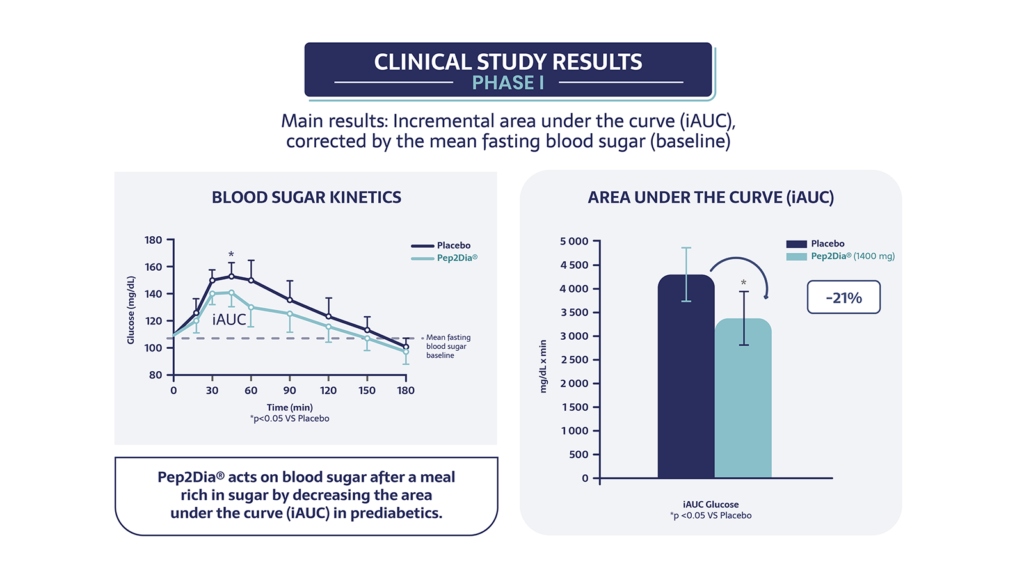
The 2020 clinical study also demonstrated a significant reduction in postprandial glycaemia at doses of 700 and 1400mg compared with placebo, in a respondent population of 15 people.
Pep2Dia® is effective on prediabetic people from a dose of 700 mg. These studies confirm that Pep2Dia® helps maintain healthy blood sugar levels after meals.
The 3rd clinical study of 2023 was conducted over 12 weeks and produced new results on glycated hemoglobin, 2h blood glucose and insulin sensitivity.
To find out more, please fill in the contact form!
Continue reading :
Contact us
Our latest publications
Want to learn more about topics related to blood sugar management?
Here are our most recent blog posts!


Contact information
51 Avenue F. Lobbedez
CS 60946
62033 Arras Cedex
France
Tel : +33 (0)3 21 23 80 00
Fax : +33 (0)3 21 23 80 01